MEDICA is the leading international platform for the medical technology industry. For more than 50 years, it has been a must-attend event in the annual calendar of trade visitors from all areas of the healthcare industry.

USTAR BIOTECHNOLOGIES(HANGZHOU)LTD will participate in this event at Booth: 03 H92-2 and look forward to meeting you.
Company Profile
USTAR BIOTECHNOLOGIES (HANGZHOU) LTD. was established in 2005, dedicated to the research, development, manufacturing and sales of innovative POCT molecular diagnostic technology and products. We have established a technical platform with completely independent IP rights, and applied for over 50 international and domestic invention patents, including 38 authorized patents. Ustar keeps participating in national projects for the prevention and control of infectious diseases, and has won dozens of national grants and awards. Our nucleic acid amplification and detection analyzer was certified by National Medical Products Administration (NMPA) through the emergency approval channel in 2019; the COVID-19 nucleic acid test kit obtained NMPA registration certificate in early 2020 as the first domestic approved listed COVID-19 molecular POCT product. Ustar’s POCT kits integrate nucleic acid extraction, purification, amplification and test in an enclosed cartridge to achieve a fast, accurate, simple and safe test with LOD≤200 copies/mL.
Ustar's products have served nearly 3,000 medical institutions and exported to over 70 countries, actively contributing to the global prevention and control of major infectious diseases such as COVID-19 and tuberculosis. Our vision is to make molecular tests available to anyone, anywhere, as stated in our slogan: “Molecular testing, anywhere!”.
Products Introduction
Product 1---PortNAT® System: Nucleic Acid Self Testing

The PortNAT® System, designed with an all-in-one test cassette and a reusable incubator, delivers fast, accurate and instrument-free nucleic acid testing in a variety of scenarios right at the point of care.
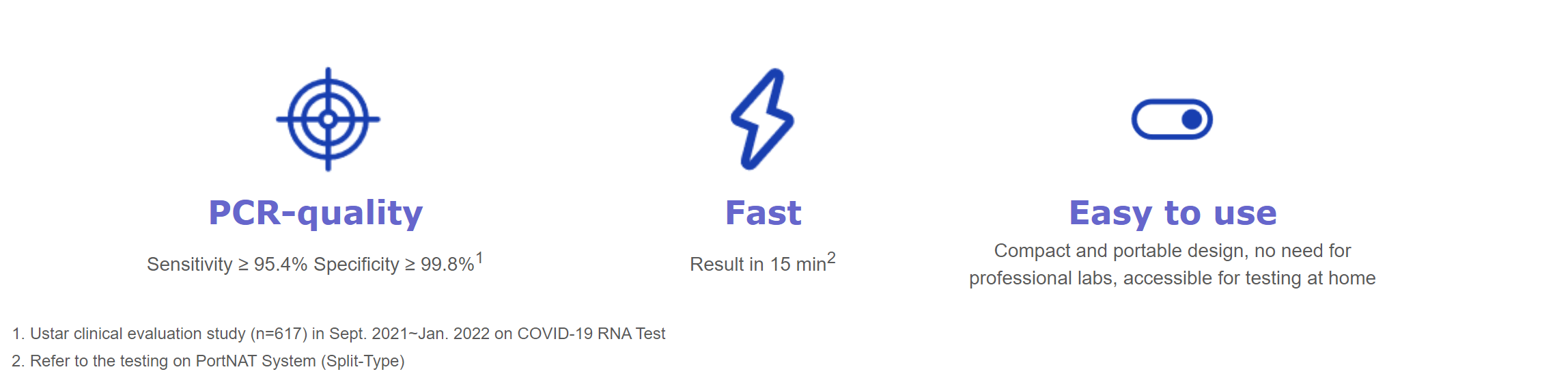
Extensive Testing on PortNAT System

Product 2---MultNAT® System: Molecular Diagnostic PCR System
The MultNAT® System, based on fluorescence polymerase chain reaction (PCR) technology, is a fully integrated and automatic system designed to meet the increasing demand for multiplex, high-throughput quantitative testing.

FDA-listed/ Bio-safe/ Multiplex/ High thoroughput
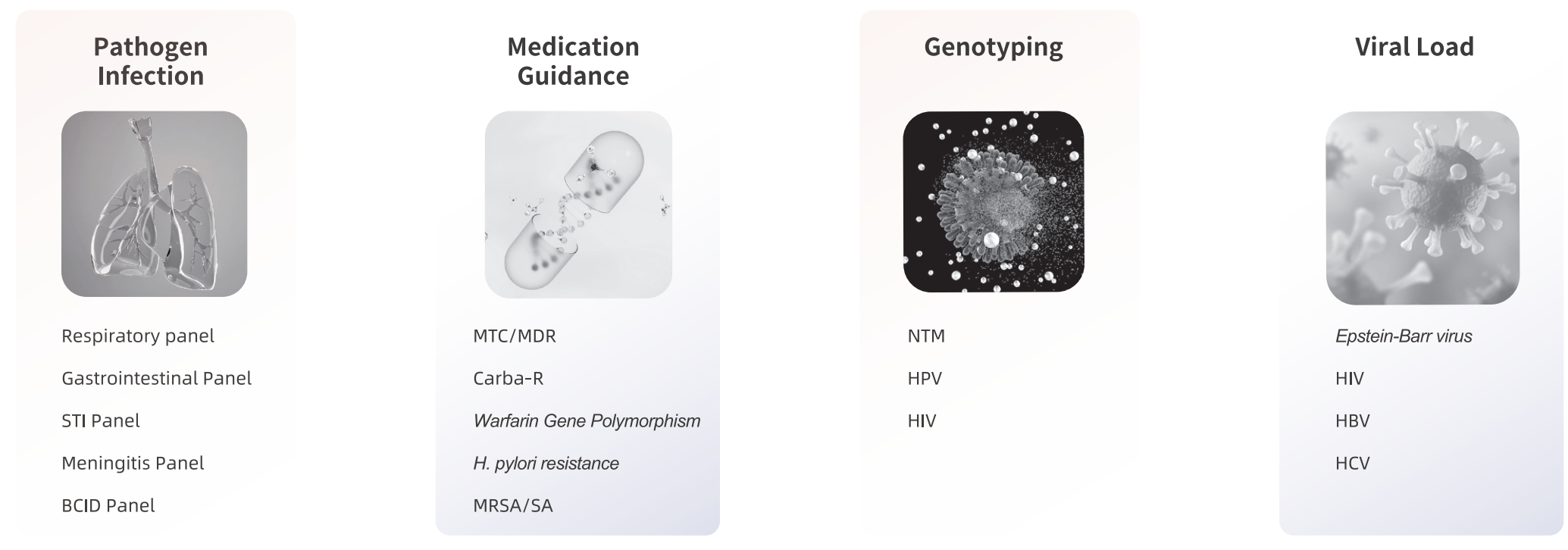
Extensive Testing on MultNAT System: